Rethinking personalized cancer therapy: targeting minimal residual disease in high-risk lymphoma patients
In this project FRRB finances the Coordinator: Istituto Europeo di Oncologia (IEO). The Principal Investigator responsible of the project is Dr. Bruno Bernardo Amati.
| Pathology of interest: | Lymphoma |
| Area of research: | Oncology/Hematology |
| Start date: | 1st August 2021 |
| End date: | 1st August 2024 |
| Funding: | € 499.900,00 |
| Project partners: | - Istituto Europeo di Oncologia (IEO) - University Hospital Essen - Charité ‐ Universitätsmedizin Berlin (CVK) - APHP, Hôpital Saint‐Louis - Centre Henri Becquerel - Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (I.R.S.T.) S.r.l. |
PROJECT SUMMARY
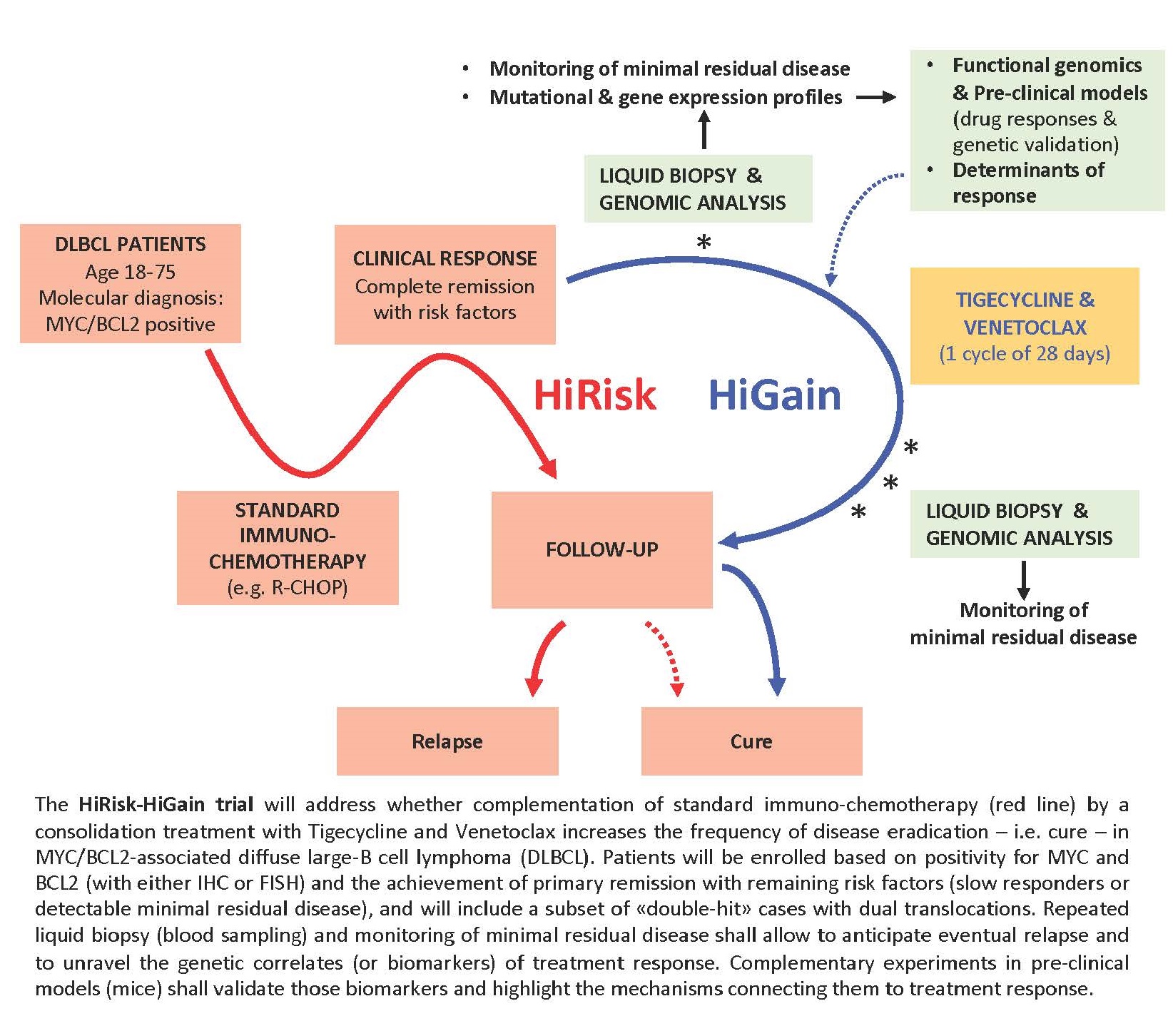
Diffuse large B‐cell lymphoma is a heterogeneous disease. 30‐40% of all patients fail induction therapy, with limited success of next‐line regimens.
Among several pathological and genetic features, deregulation of the MYC and BCL2 oncogenes is a strong predictor of negative prognosis: indeed, overexpression of their protein products ‐ routinely assayed by immunohistochemistry ‐ allows detection of “double‐expresser” lymphomas (DEL), representing a sizeable patient population with an acute unmet medical need.
Classically, personalized medicine approaches would either aim at identifying actionable lesions at relapse, or utilize information available at diagnosis to expand the induction regimen with targeted therapy.
Here, project partnership proposes to “re‐think” personalized medicine with an innovative phase I/II trial, aimed at eradicating minimal residual disease (MRD) in DEL patients following a clinical response to induction immune‐chemotherapy.
Toward this aim, project partnership will use a targeted drug combination (Venetoclax and Tigecycline, or V/T) with demonstrated efficacy against BCL2/MYC DEL cells.
At the various stages, liquid biopsies will be used to assess the presence of MRD, as well as to establish full mutational and expression profiles, thus allowing us to decipher a posteriori the molecular underpinnings that characterize responders and non‐responders.
This will be complemented with a systematic effort to optimize health care by defining conceptually novel stratification criteria for the V/T consolidation regimen, exemplifying a fundamentally novel route into personalized medicine in the clinic.
Finally, clinical activities will be complemented by a focused pre‐clinical research plan, in which mouse models that recapitulate high‐risk DLBCL subsets will be subject to similar sequential regimens, thereby addressing the mechanisms of action of the relevant drugs, as well as the functional implications of defined mutational and transcriptional profiles.